


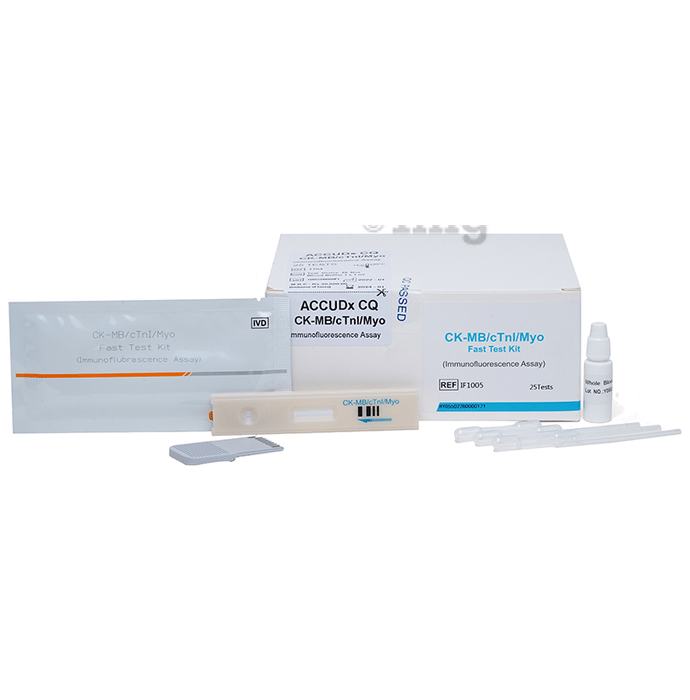
ACCUDx CQ CK-MB/c Tnl/Myo Immunofluorescence Assay
Product highlights
- Intended for in vitro quantitative determination of CK-MB/cTnl/Myoin serum, plasma or whole blood
- This test is used as an aid in the clinical diagnosis and prognosis
- Evaluation of myocardial injury such as acute myocardial infarction (ami), unstable angina, acute myocarditis and acute coronary syndrome
MRP₹300003% off
₹29100
Inclusive of all taxes
of25 Test Kits
Get it delivered by Saturday, 15 March
Delivering to:
Additional offers
Amazon Pay: Pay with Amazon Pay Balance and get cashback up to Rs. 100 with minimum cashback of Rs. 20. Offer ends 31st Mar'25. Minimum cart value to avail the offer is Rs. 699. Reward is available behind scratch card on Amazon Pay.
Information about ACCUDx CQ CK-MB/c Tnl/Myo Immunofluorescence Assay
ACCUDx CQ CK-MB/c Tnl/Myo Immunofluorescence Assay
Mixed monoclonal antibodies against human CK-MB, cT and Myo are conjugated with fluorescence latex, and another set of anti-human CK-MB/cTnI/Myo monoclonal antibodies were coated on different test lines, respectively. After the sample has been applied to the test strip, the fluorescence latex-labelled anti-human CK-MB, cTnl and monoclonal antibodies will bind with the CK-MB, cTnl and Myo in sample, respectively and form marked antigen-antibody complexes. These complexes move to the test card detection zone by capillary action. Then marked antigen-anti- body complexes will be captured on different test lines by another set of monoclonal antibodies against human CK-MB, cT or Myo, respectively resulting in the accumulation of fluorescence particles on the test lines. The fluorescence intensity of each test line increases in proportion to the amount of CK-MB, cTnl or Myo in sample. Then insert test card into ACCUDx CQ Immunofluorescence Quantitative Analyzer, the concentration of CK-MB, cT and Myo in sample will be measured and displayed on the screen. The value will be stored in ACCUDX CQ Analyzer and available for downloading. The result can be easily transmitted to LIS and HIS.
Uses:
This test is used as an aid in the clinical diagnosis, prognosis and evaluation of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome
Product Specifications and Features:
Directions For Use:
Safety Information:
Mixed monoclonal antibodies against human CK-MB, cT and Myo are conjugated with fluorescence latex, and another set of anti-human CK-MB/cTnI/Myo monoclonal antibodies were coated on different test lines, respectively. After the sample has been applied to the test strip, the fluorescence latex-labelled anti-human CK-MB, cTnl and monoclonal antibodies will bind with the CK-MB, cTnl and Myo in sample, respectively and form marked antigen-antibody complexes. These complexes move to the test card detection zone by capillary action. Then marked antigen-anti- body complexes will be captured on different test lines by another set of monoclonal antibodies against human CK-MB, cT or Myo, respectively resulting in the accumulation of fluorescence particles on the test lines. The fluorescence intensity of each test line increases in proportion to the amount of CK-MB, cTnl or Myo in sample. Then insert test card into ACCUDx CQ Immunofluorescence Quantitative Analyzer, the concentration of CK-MB, cT and Myo in sample will be measured and displayed on the screen. The value will be stored in ACCUDX CQ Analyzer and available for downloading. The result can be easily transmitted to LIS and HIS.
Uses:
This test is used as an aid in the clinical diagnosis, prognosis and evaluation of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome
Product Specifications and Features:
- It is intended for in vitro quantitative determination of CK-MB/cTnl/Myoin serum, plasma or whole blood
- This test is used as an aid in the clinical diagnosis, prognosis and evaluation of myocardial injury such as Acute Myocardial Infarction (AMI), Unstable Angina, Acute Myocarditis and Acute Coronary Syndrome
Directions For Use:
- Collect specimen according to manual
- Test card, sample and reagent should be brought to roomtemperatureprior to testing
- Confirm SD card lot No. in accordance with test kit lot No.. Perform“SDCard Calib” calibration when necessary
- On the main interface of Getein1100, press ""ENT"" button to enter testinginterface
- Remove the test card from the sealed pouch immediately beforeuse. Label the test card with patient or control identification
- Put the test card on a clean table, horizontally placed
- Using sample transfer pipette, deliver 100 μl of sample into onetubeof sample diluent, mix gently and thoroughly. Then drop 100 μl (or 3dropsof sample when using disposable pipet) of sample mixture into thesampleport on the test card
- After 10 minutes, insert the test card into Getein1100 and press ""ENT""button. The result will be shown on the screen
Safety Information:
- For in vitro diagnostic use only
- For professional use only
- Do not use the kit beyond the expiration date
- Do not use the test card if the foil pouch is damaged
- Do not open pouches until ready to perform the test
- Do not reuse the test card
- Do not reuse the pipet
- Handle all specimens as potentially infectious
- Proper handling anddisposal methods should be followed in accordance with local regulations
- Carefully read and follow the manual to ensure proper test performance
Country of origin: China
Expires on or after: Not Applicable
A licensed vendor partner from your nearest location will deliver ACCUDx CQ CK-MB/c Tnl/Myo Immunofluorescence Assay. Once the pharmacy accepts your order, the details of the pharmacy will be shared with you.
Phone Number: 0124-4166666
Address: 5th Floor Tower - B of the Presidency Building, 46/4 Mehrauli Gurgaon Road, Sector 14, Gurugram, Haryana-122001, India
Expires on or after: Not Applicable
A licensed vendor partner from your nearest location will deliver ACCUDx CQ CK-MB/c Tnl/Myo Immunofluorescence Assay. Once the pharmacy accepts your order, the details of the pharmacy will be shared with you.
In case of any issues, contact us
Email ID: care@1mg.comPhone Number: 0124-4166666
Address: 5th Floor Tower - B of the Presidency Building, 46/4 Mehrauli Gurgaon Road, Sector 14, Gurugram, Haryana-122001, India
 Authentic Products
Authentic Products Great Savings
Great Savings Home Delivery
Home Delivery





